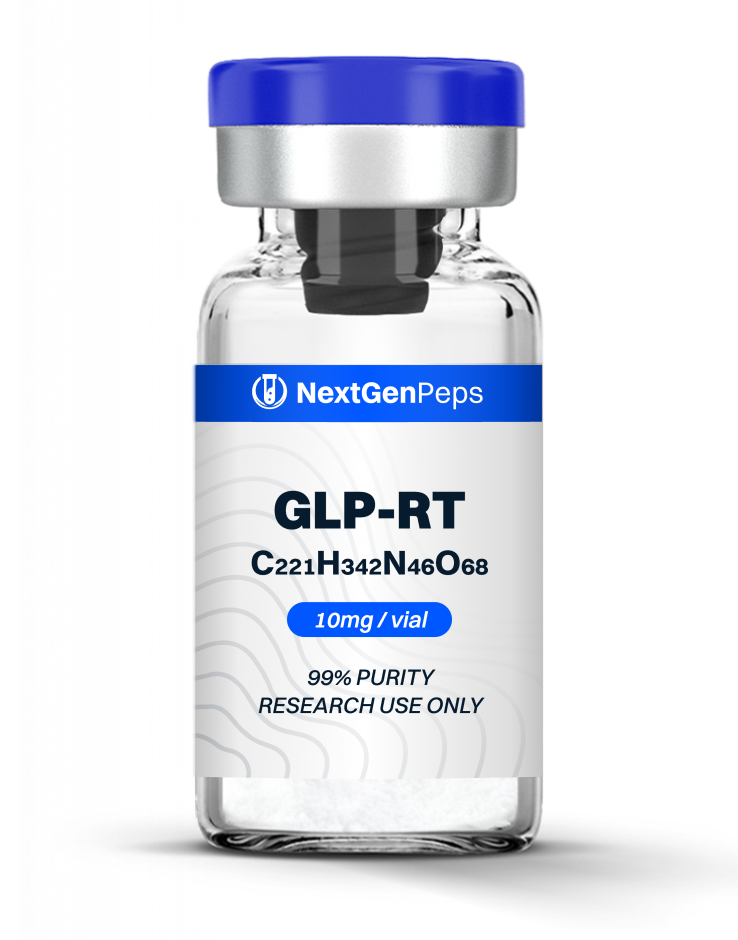
GLP-RT
Size:
GLP-RT Overview
GLP-RT (LY3437943) is a high-purity, research-grade synthetic peptide developed as a triple receptor agonist that simultaneously targets GLP‐1, GIP, and glucagon receptors. Its single, continuous helical conformation and long plasma half‐life make it a central tool for modeling intensive incretin/glucagon‐based metabolic interventions.
Molecular Composition and Mechanistic Profile
GLP-RT adopts a single continuous helix where:
The N‐terminal segment engages the receptor transmembrane domain.
The C‐terminal segment interacts with extracellular regions of GLP‐1 and GIP
receptors.
It exhibits the following potencies (EC50):
GIP receptor: 0.0643 nM
GLP‐1 receptor: 0.775 nM
Glucagon receptor: 5.79 nM
The peptide shows a terminal half‐life of ~6 days, undergoes primarily hepatic metabolism, and shows little interaction with cytochrome P450 enzymes.
Integrated Research Applications
GLP-RT is used to study intensive metabolic modulation across several domains:
Obesity and weight loss: Phase 2 trials in obesity show robust, dose‐dependent reductions in body weight, reflecting combined appetite suppression, delayed gastric emptying, and increased energy expenditure.
Glycemic control and diabetes: Triple agonism allows investigation of potent HbA1c lowering, fasting and post‐prandial glucose improvements, and β‐cell function compared with single or dual incretin agents.
Metabolic liver disease: Randomized trials in metabolic dysfunction–associated steatotic liver disease (MASLD/MASH) evaluate its impact on liver fat content, inflammation, and fibrosis markers.
Kidney and cardio‐metabolic protection: Comparative animal studies against liraglutide and tirzepatide explore effects on diabetic kidney disease and broader cardio‐renal endpoints.
Ligand–receptor structural work clarifies how GLP-RT simultaneously engages GLP‐1R, GIPR, and GCGR to create a distinctive metabolic signature, including reductions in gastric emptying rate, food intake, and body‐weight set‐point.
Analytical Validation, Formulation, and Storage
HPLC purity: 99.4–99.5%.
Source: Fully synthetic; supplied as lyophilized powder.
Counterion: Typically supplied as a sodium salt; TFA counterion from HPLC
purification may be present and can contribute to total mass and influence solubility.
Storage and handling guidelines:
Delivered lyophilized; store at or below −20 °C to maintain long‐term stability.
Reconstitute with appropriate sterile buffer for in vitro or in vivo experimental work, following standard peptide‐handling SOPs. Residual TFA:
TFA salt can account for up to ~20% of total mass; peptide content is generally ≥80% of the net weight.
TFA typically improves aqueous solubility and does not interfere with standard in vitro assays, though highly sensitive assays should consider its presence.
NextGenPeps supplies GLP-RT exclusively for laboratory research in vitro or in approved animal models. This compound is not approved for human use and is not marketed as a drug, supplement, or diagnostic agent.
Frequently Asked Questions
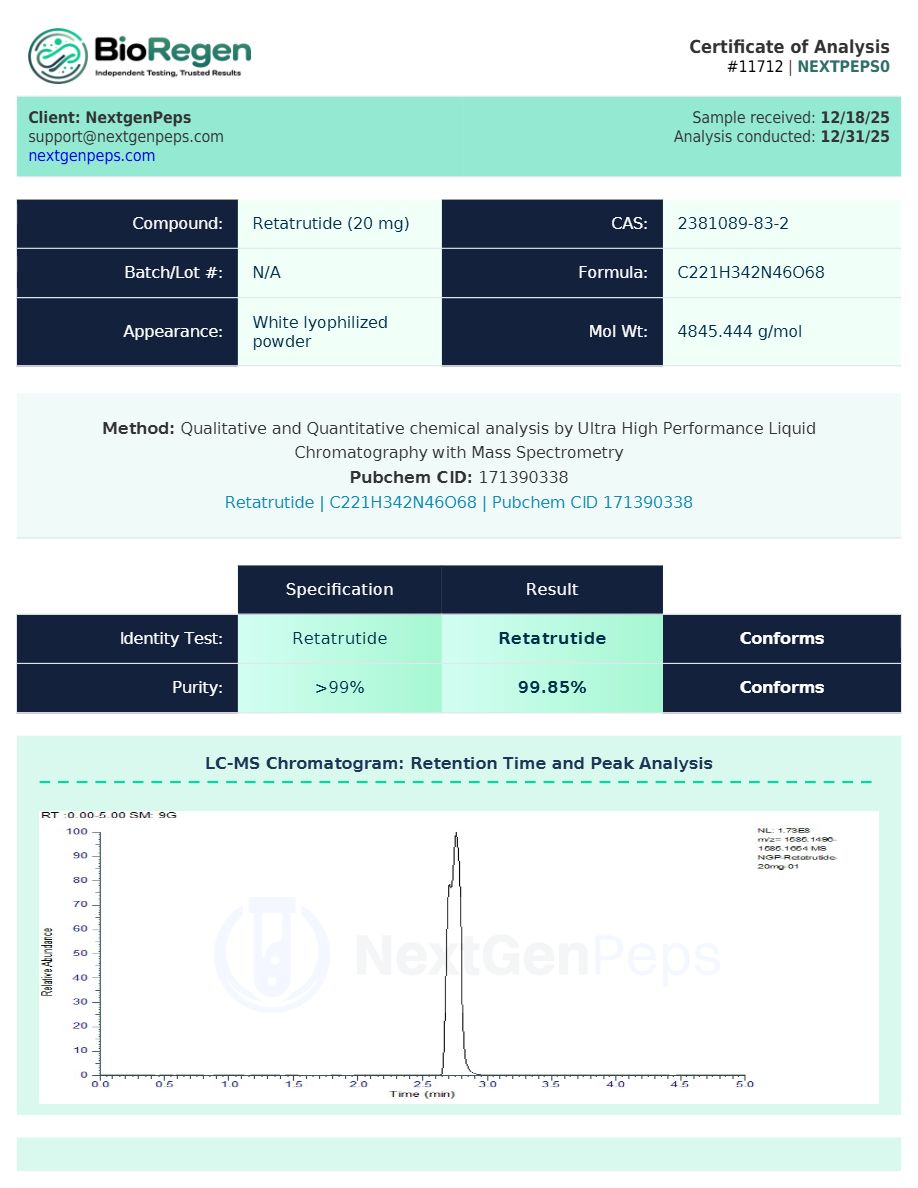
Test Results 20MG
Qualitative and Quantitative chemical analysis for GLP-RT 20MG by Ultra High Performance Liquid Chromatography with Mass Spectrometry
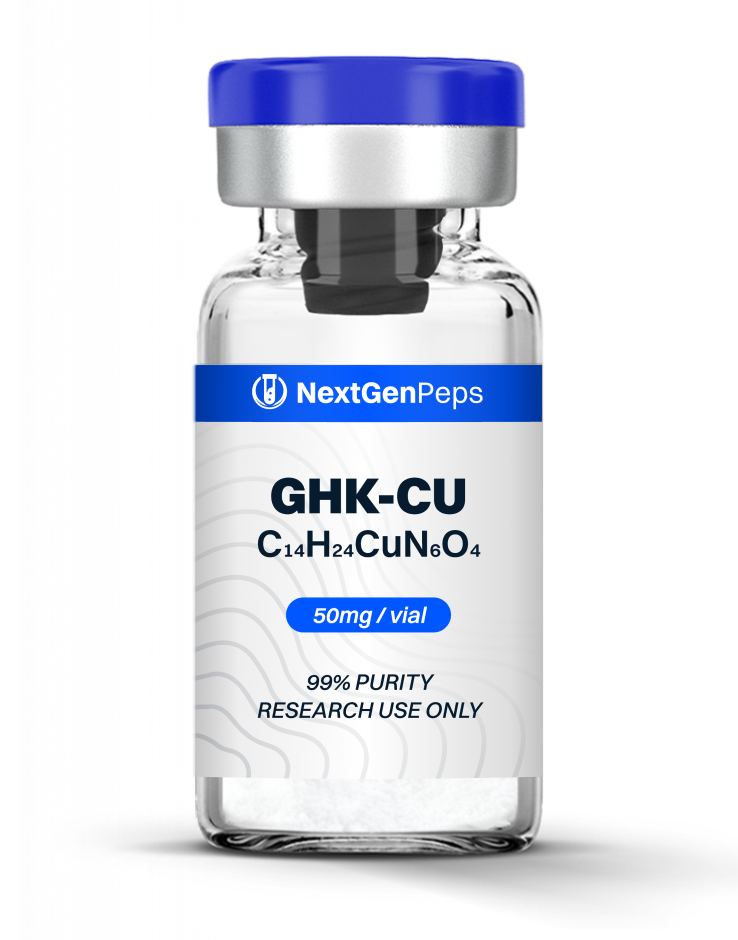
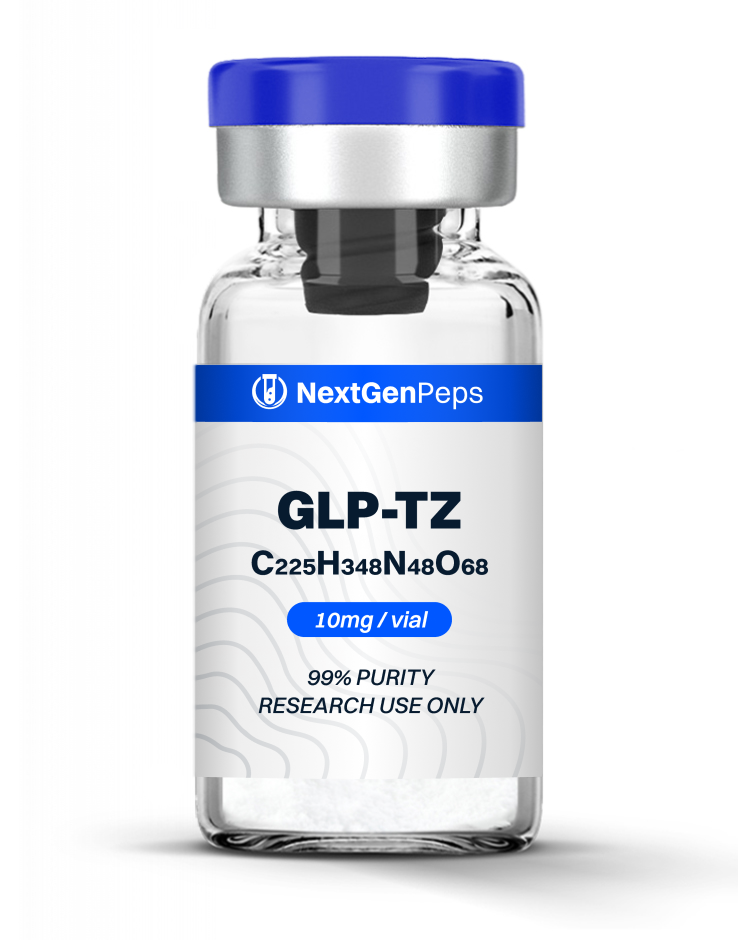
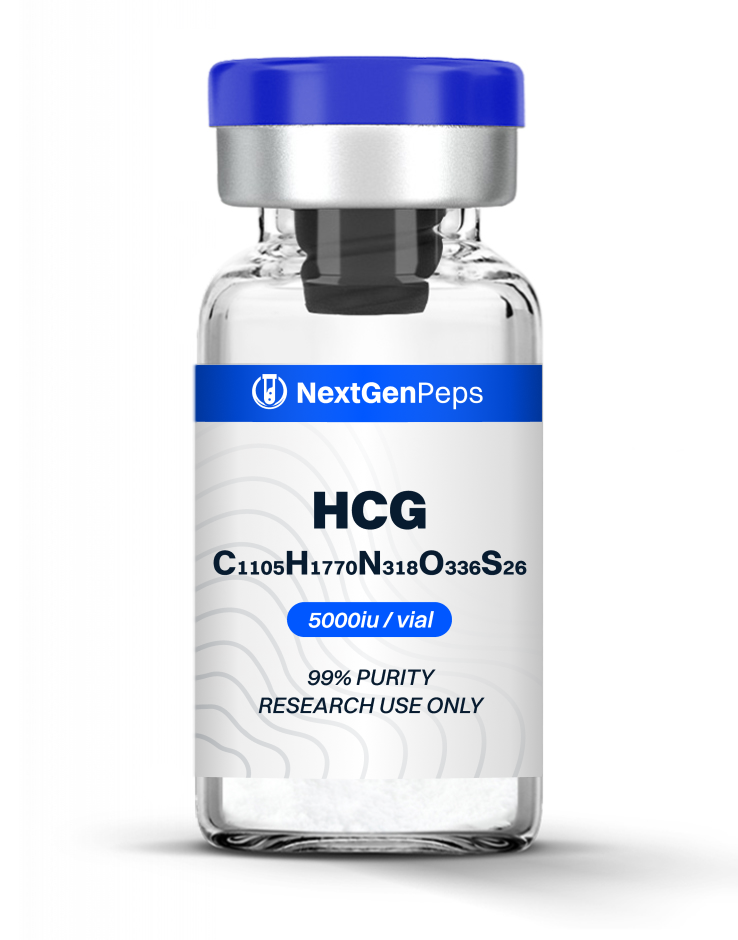
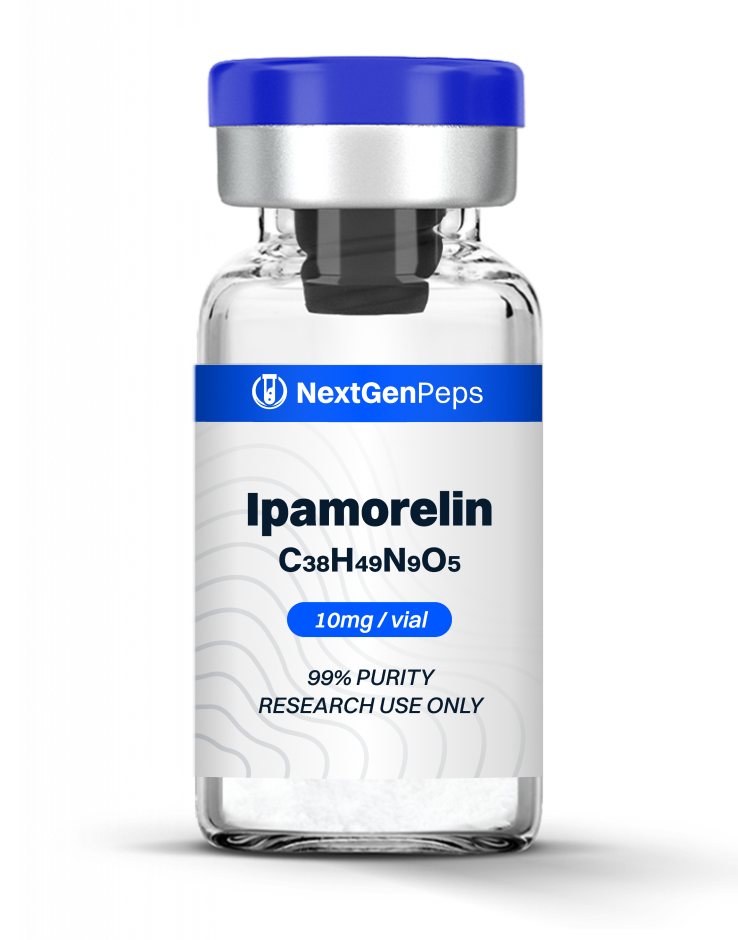
Similar Products
Reliable and innovative research with our top-grade research materials and novel research compounds tailored to lead the competitive research industry. Choose research quality. Choose NextGenPeps.
Get 10% Off Your First Order
Sign up now and receive a 10% discount code valid on your next purchase!
NextGenPeps LLC | 1331 Columbia Street, San Diego, CA 92101 | Copyright 2025 NextGenPeps
The statements made within this website have not been evaluated by the US Food and Drug Administration. The statements and the products of this company are not intended to diagnose, treat, cure or prevent any disease. All Peptide research products on this site are for research and development use only, and not intended for human use.
NextGenPeps is a chemical supplier. NextGenPeps is not a compounding pharmacy or chemical compounding facility as defined under 503A of the Federal Food, Drug, and Cosmetic act. NextGenPeps is not an outsourcing facility as defined under 503B of the Federal Food, Drug, and Cosmetic Act.