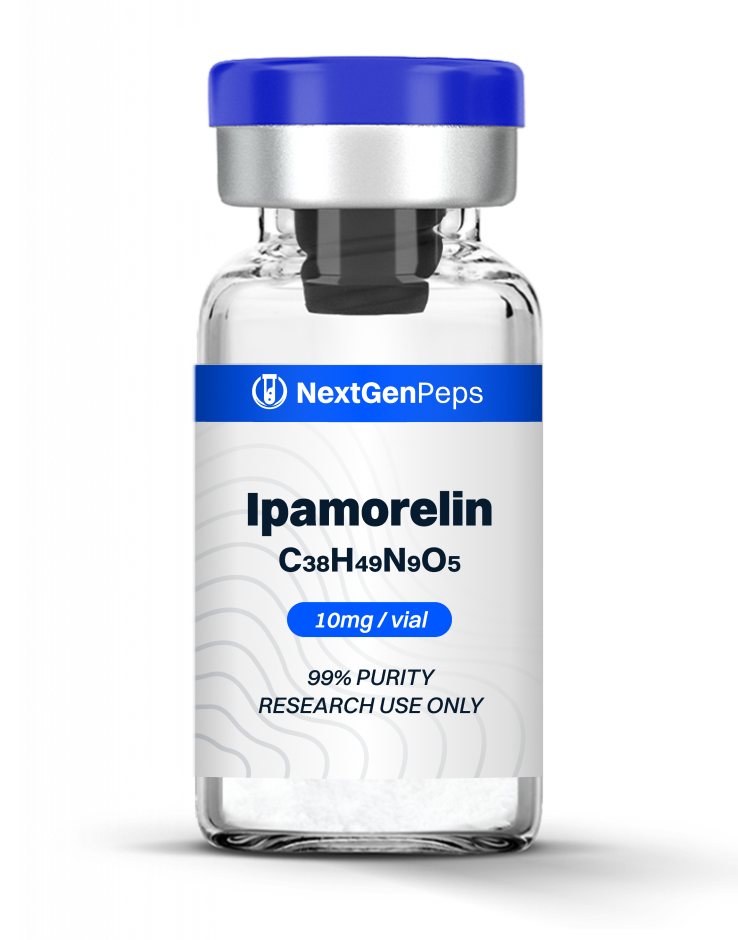
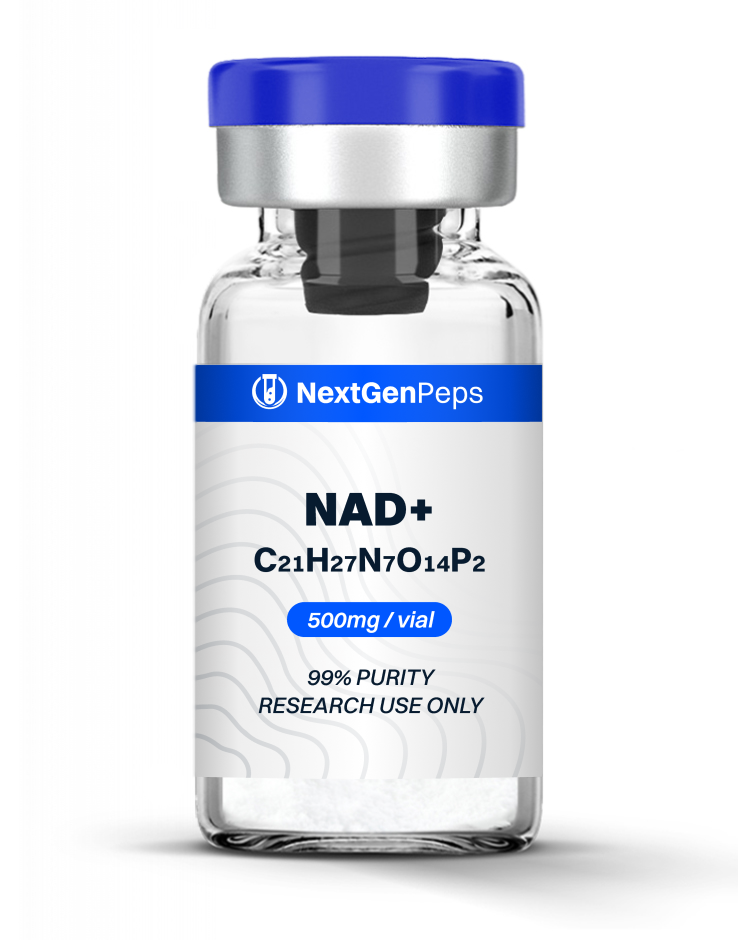
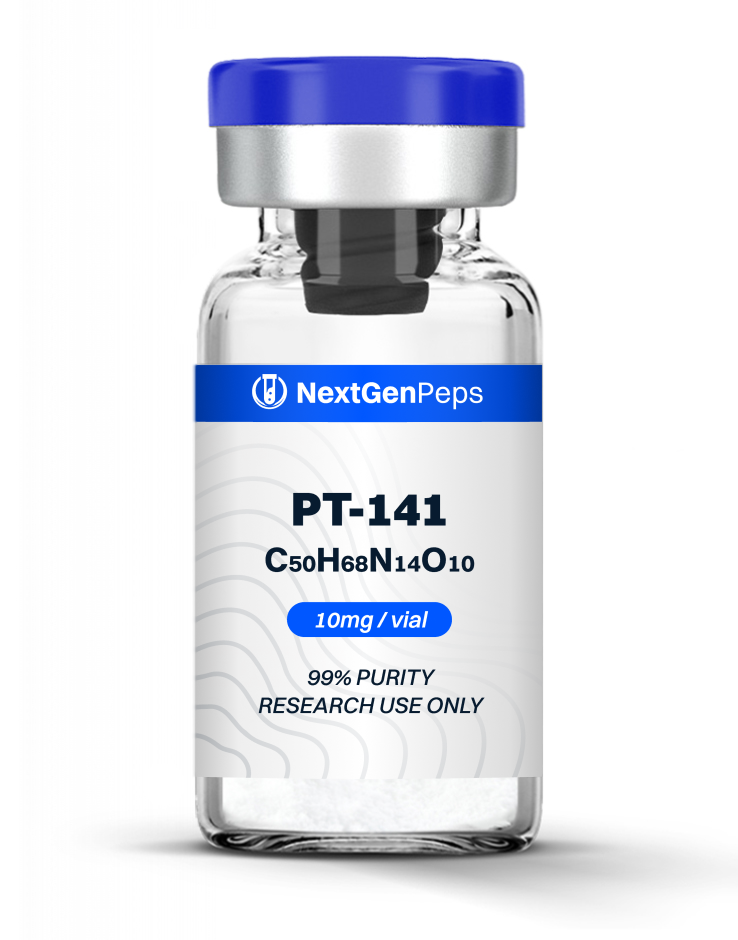
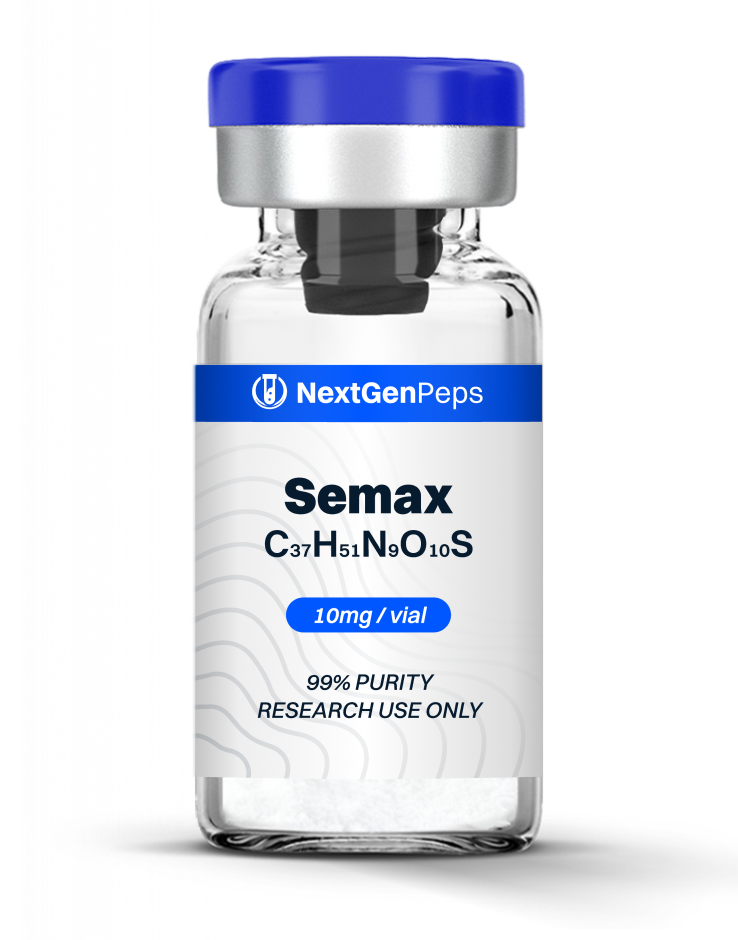
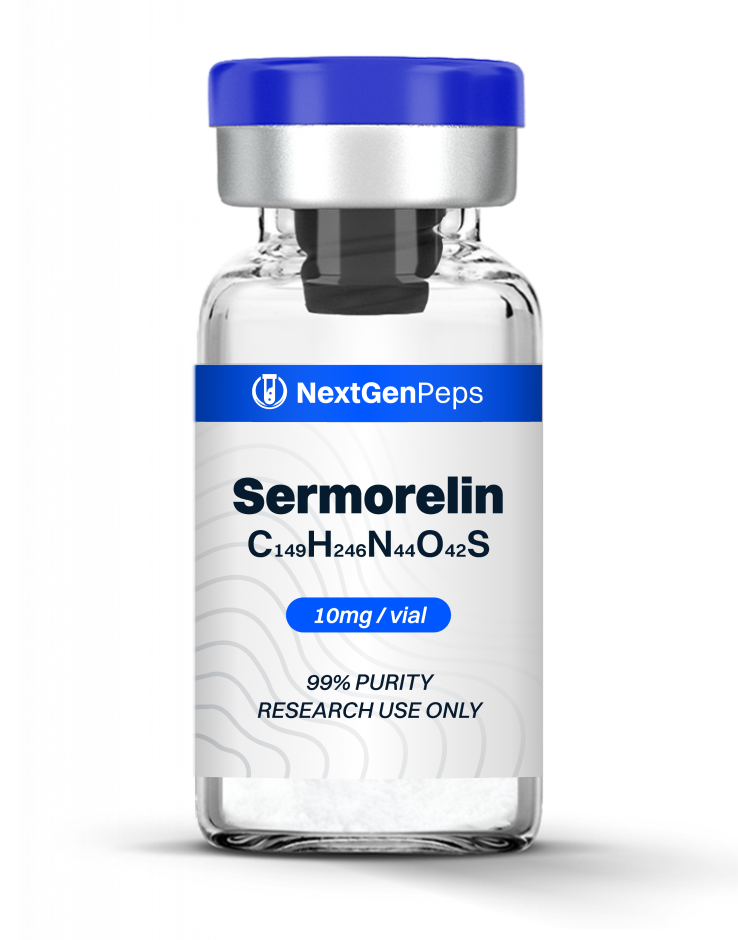
TB-500
TB‐500 Fragment (17‐23), also known as fequesetide, is a high‐purity, research-grade heptapeptide derived from the active domain of thymosin beta‐4 (TB4), engineered to model actin regulation, cell migration, and tissue repair in controlled experimental systems. This fragment represents the minimal TB4 sequence that retains the parent protein’s core binding and cytoskeletal effects while improving stability and bioavailability.
Molecular Composition and Mechanistic Profile
Mechanistically, TB‐500 Fragment (17‐23):
Binds directly to actin, modulating the balance between monomeric G‐actin and filamentous F‐actin and thereby influencing cell structure, motility, and replication.
Functions as a short, cell‐penetrating peptide capable of crossing cell and even nuclear membranes, which supports efficient intracellular and intranuclear access in vitro and in vivo.
Modulates signaling pathways such as Akt and Bcl‐XL, which are involved in cell survival, anti‐apoptotic responses, and tissue recovery.
Compared with full‐length TB‐500, the fragment shares the same peptide sequence (LKKTETQ) but lacks an aldehyde group on the leucine residue, a difference that reduces its molecular weight (889.0 vs 846.97 g/mol) and is proposed to increase stability, prolong half‐life, and reduce off‐target interactions.
Integrated Research Applications
TB‐500 Fragment (17‐23) is employed to dissect TB4‐related repair mechanisms with a more focused, fragment‐based tool. Representative research domains include:
Wound healing and soft‐tissue repair: Animal models show accelerated wound closure, reduced inflammation, enhanced blood vessel growth (angiogenesis), and decreased scar formation, driven in part by fibroblast migration and cytoskeletal reorganization.
Muscle injury and regeneration: In muscle‐damage models, TB‐500 Fragment (17‐23) activates satellite cells, promotes their proliferation, and supports regeneration of damaged myofibers, likely via Akt‐mediated signaling and enhanced cell motility.
Musculoskeletal function and performance: Studies suggest improvements in musculoskeletal recovery and performance metrics by accelerating repair of micro‐injury from training and supporting structural integrity of muscle and connective tissues.
Neuroinflammation and CNS repair: TB4’s benefits in the brain, such as support for remyelination and neuroprotection, appear to be mediated through the same active domain represented by TB‐500 Fragment (17‐23), suggesting similar potential roles in CNS repair models.
Autoimmune demyelinating disease models (e.g., MS): By dampening inflammatory processes involved in myelin damage, TB‐500 Fragment (17‐23) has been proposed as a tool to study prevention of demyelination and relapse dynamics in multiple sclerosis–like models.
These actin‐modulating capacity to explore both local and systemic tissue repair mechanisms.
applications leverage the fragment’s small size, membrane permeability, and
Analytical Validation and Quality Features
TB‐500 Fragment (17‐23) is supplied by research vendors as a lyophilized powder at defined peptide content per vial (e.g., 10 mg). Typical analytical and manufacturing specifications include:
Purity: ≥99% as confirmed by high-performance liquid chromatography (HPLC) and/or mass spectrometry (MS).
Identity: Verified via MS and peptide sequencing to confirm the LKKTETQ heptapeptide structure and correct molecular weight.
Manufacturing standards: Often produced in ISO 9001 and cGMP‐audited facilities, with certificates of analysis (COAs) available on request.
These controls support batch‐to‐batch consistency, enabling reliable comparison of results across studies and laboratories.
Storage and Stability Guidelines
Although exact storage instructions can vary by supplier, standard peptide handling practices apply to TB‐500 Fragment (17‐23).
Lyophilized peptide: Store in a cool, dry place, protected from light, typically at refrigerated or frozen temperatures (e.g., ≤ −20 °C) for long‐term stability.
After reconstitution: Maintain at low temperature (refrigerated), minimize freeze–thaw cycles, and use within a defined experimental window according to institutional SOPs.
These conditions help preserve peptide integrity, limit degradation, and maintain consistent bioactivity in ongoing research.
NextGenPeps offers TB-500 strictly for controlled laboratory research purposes. This peptide is not approved for therapeutic or diagnostic use and is not intended for human consumption, clinical treatment, or use outside approved experimental protocols.
Frequently Asked Questions
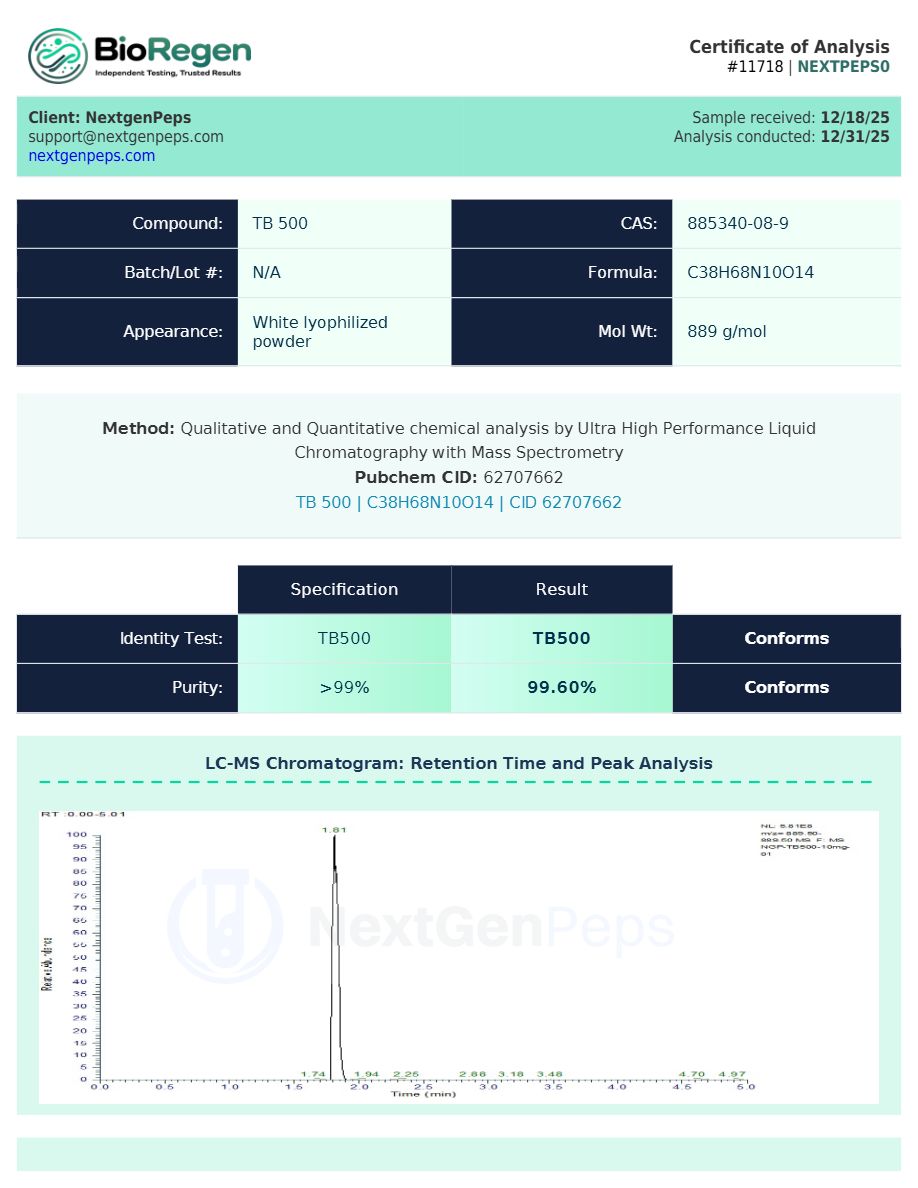
Test Results 10mg
Qualitative and Quantitative chemical analysis for TB-500 10mg by Ultra High Performance Liquid Chromatography with Mass Spectrometry
Similar Products
Reliable and innovative research with our top-grade research materials and novel research compounds tailored to lead the competitive research industry. Choose research quality. Choose NextGenPeps.
Get 10% Off Your First Order
Sign up now and receive a 10% discount code valid on your next purchase!
NextGenPeps LLC | 1331 Columbia Street, San Diego, CA 92101 | Copyright 2025 NextGenPeps
The statements made within this website have not been evaluated by the US Food and Drug Administration. The statements and the products of this company are not intended to diagnose, treat, cure or prevent any disease. All Peptide research products on this site are for research and development use only, and not intended for human use.
NextGenPeps is a chemical supplier. NextGenPeps is not a compounding pharmacy or chemical compounding facility as defined under 503A of the Federal Food, Drug, and Cosmetic act. NextGenPeps is not an outsourcing facility as defined under 503B of the Federal Food, Drug, and Cosmetic Act.